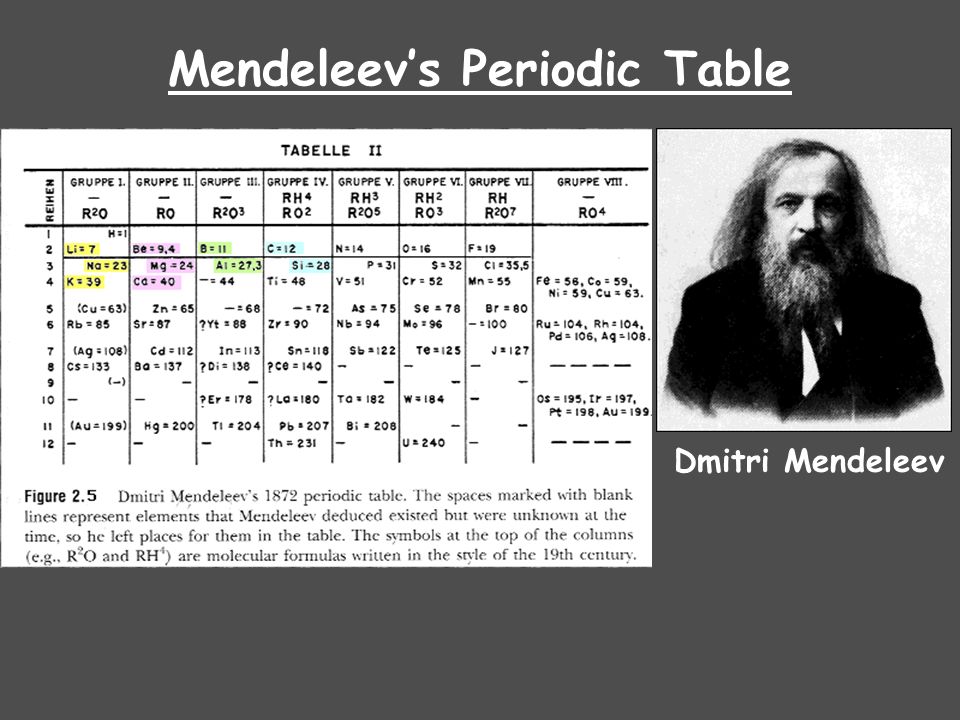
Remarkable features about Mendeleev’s periodic law are:
(i) Basis of classification:
Elements were grouped on a more fundamental basis than earlier attempts.
ADVERTISEMENTS:
(ii) Prediction of properties of new elements:
Because of their placement in the periodic table, properties of several yet to be discovered elements like scandanium, gallium and germanium could be predicted.
(iii) Correction of atomic weights/masses:
ADVERTISEMENTS:
Mendeleev gave more weight age to similarities in properties of elements rather than atomic masses. So, atomic masses of some elements got corrected. Atomic weights of elements like gold and platinum could thus be corrected.
(iv) Inert gases:
The discovery of new elements, inert gases, did not disturb the arrangement and they found their place as a new group.
(v) Gaps for 40 new elements were kept.