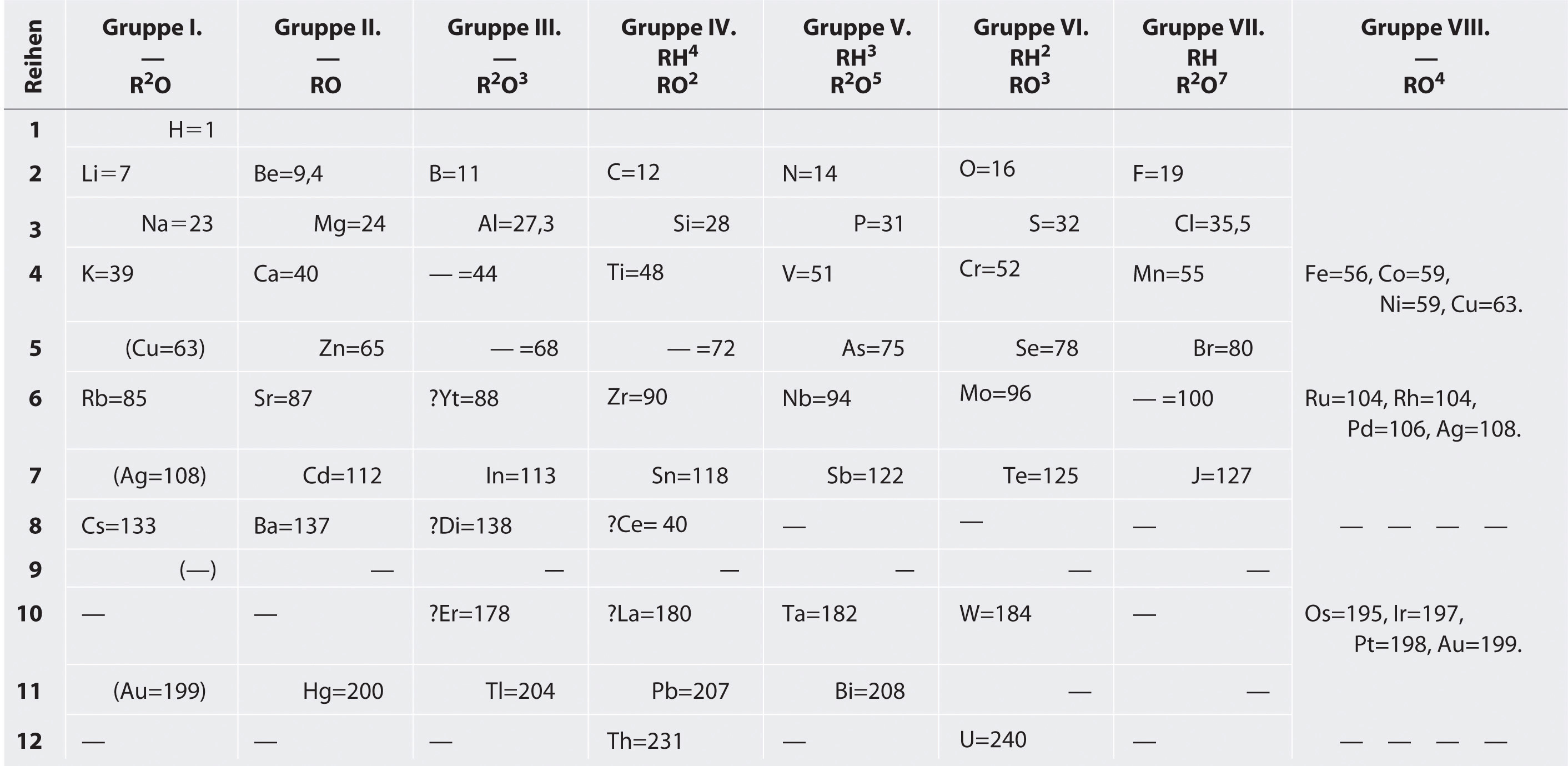
Because of placement of elements in increasing atomic masses in Mendeleev’s periodic table, certain chemically similar elements were separated and chemically dissimilar elements were to be grouped together.
For example, Argon with atomic mass 39.948 needs to be placed after potassium with atomic mass 39.0983.
Nickel with atomic mass 58.69 was placed before cobalt with atomic mass 58.93. But as per their chemical properties, these are placed in reversed order.
ADVERTISEMENTS:
As the modern law is based on atomic number or number of electrons, elements with similar valence electrons fall in the same group.
Since the chemical properties of elements depend on their valence electrons, anomalies in the Mendeleev’s table are automatically removed.
Further placement of hydrogen with alkali metals is now justified on the basis of its electronic configuration.