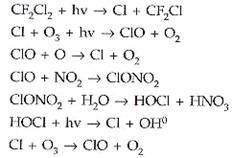The term Ozone depletion is used to describe two distinct, but related, observations: a slow, steady decline, of about 3% per decade, in the total amount of ozone in the Earth’s stratosphere during the past twenty years, and a much larger, but seasonal, decrease in stratospheric ozone over the Earth’s polar regions during the same period.
(The latter phenomenon is commonly referred to as the “ozone hole“.) The detailed mechanism by which the polar ozone holes form is different from that for the mid-latitude thinning, but the proximate cause of both trends is believed to be catalytic destruction of ozone by atomic chlorine and bromine.
The primary source of these halogen atoms in the stratosphere is photodissociation of chlorofluorocarbon (CFC) compounds, commonly called Freons, and bromofluorocarbon compounds known as Halons, which are transported into the stratosphere after being emitted at the surface. Both ozone depletion mechanisms strengthened as emissions of CFCs and Halons increased.
ADVERTISEMENTS:
Since the ozone layer prevents most harmful UVB wavelengths (270- 315 nm) of ultraviolet light from passing through the Earth’s atmosphere, observed and projected decreases in ozone have generated worldwide concern, leading to adoption of the Montreal Protocol banning the production of CFCs and halons as well as related ozone depleting chemicals such as carbon tetrachloride and trichloroethane (also known as methyl chloroform).
It is suspected that a variety of biological consequences, including, for example, increases in skin cancer, damage to plants, and reduction of plankton populations in the ocean’s photic zone, may result from the increased UV exposure due to ozone depletion.
Ozone Creation:
Three forms (or allotropes) of oxygen are involved in the ozone-oxygen cycle Oxygen atoms or atomic oxygen, O, oxygen molecules, O2 and ozone, O3. Ozone is formed in the stratosphere when oxygen molecules photo dissociate after absorbing an ultraviolet photon whose wavelength is shorter than 240 nm.
ADVERTISEMENTS:
This produces two oxygen atoms. The atomic oxygen then combines with O2 to create O3. Ozone molecules strongly absorb UV light between 310 and 200 nm, following which ozone splits into a molecule of O2 and an oxygen atom. The oxygen atom then joins up with an oxygen molecule to regenerate ozone.
This is a continuing process which terminates when an oxygen atom “recombines” with an ozone molecule to make 2 O2 molecules. Prior to the beginning of the depletion trend, the amount of ozone in the stratosphere was kept roughly constant by a balance between the rates of creation and destruction of ozone molecules by UV light.
O2 + hv –» O + O
O2 + O +M –» O3 + M
ADVERTISEMENTS:
O3 + hv –» O2 + O
Ozone Destruction:
Ozone can be destroyed by a number of free radical catalysts, the most important of which are hydroxyl (OH), nitric oxide (NO), atomic chlorine (CI) and bromine (Br). All of these radicals have both natural and anthropogenic (marunade) sources.
At the present time, most of the OH and NO in the stratosphere is of natural origin, but human activity has dramatically increased the chlorine and bromine. These elements are found in certain stable organic compounds, especially chlorofluorocarbons (CFCs), which may find their way to the stratosphere without being destroyed in the troposphere.
Once in the stratosphere, the CI and Br atoms are liberated from the parent compounds by the action of ultraviolet light, and can destroy ozone molecules in a catalytic cycle. In this cycle, a chlorine atom reacts with an ozone molecule, taking an oxygen atom with it (forming CIO) and leaving a normal oxygen molecule.
A free oxygen atom then takes away the oxygen from the CIO, and the final result is an oxygen molecule and a chlorine atom, which then re-initiates the cycle. The chemical shorthand for these reactions are:
For this mechanism to operate there must be a source of O atoms, which is primarily the photo dissociation of O3.
A single chlorine atom would keep on destroying ozone for upto two years (the time scale for transport back down to the troposphere) were it not for reactions that remove them from this cycle by forming reservoir species such as hydrochloric acid and chlorine nitrate.
On a per atom basis, bromine is even more efficient than chlorine at destroying ozone, but there is much less bromine in the atmosphere at present. As a result, both chlorine and bromine contribute significantly to the overall ozone depletion.