Here is an essay on ‘Air Pollution’ for class 9, 10, 11 and 12. Find paragraphs, long and short essays on ‘Air Pollution’ especially written for school and college students.
Essay on Air Pollution
Essay Contents:
- Essay on the Introduction to Air Pollution
- Essay on the Classification of Air Pollutants
- Essay on the Causes of Air Pollution
- Essay on the Forms of Air Pollution
- Essay on the Effect on Vision Resulting in the Reduction of Visibility
- Essay on the Sources of Air Pollution
- Essay on the Air Pollution in India
- Essay on the Prevention and Control of Air Pollution
Essay # 1.Introduction to Air Pollution:
Air is one of the five essentials (air, water, food, heat, and light) for the human beings. Man breaths nearly 22,000 times in a day and inhales approximately 15kg of air per day. Generally human beings can live for 5 weeks without any food, 5 days without any water but not even 5 minutes without air. Even though the air is abundantly available over the surface of the earth, but it contains lot of impurities. All the impurities in the inhaled air do not cause injury to health and it depends on several factors. Due to industrialisation in a region, the purity of air has been reducing in that locality to a level which endanger the health of the community.
ADVERTISEMENTS:
Various types of contaminants are entering into the atmosphere, by natural and man-made activities which are taking place on the earth. So, in general, air pollution means the presence of a foreign matter in air.
Various authors defined ‘air pollution’ which are as follows:
1. Dean. E.Painter defined ‘air pollution’ as the presence in the outdoor atmosphere of one or more contaminants in a sufficient quantity and duration to cause them to be injurious to human health and welfare and animal and plant life and to interfere with the enjoyment of life and property.
2. The American Medical Association, Council of Industrial Health (WHO) defined ‘air pollution’ as the excessive concentration of foreign matter in the air which adversely affects the well-being of the individual or causes damage to property.
ADVERTISEMENTS:
3. The Bureau of Indian Standards (IS: 4167) states that the pollution is the presence in ambient atmosphere of substances generally resulting from the activities of man, in sufficient concentration present for a sufficient time and under circumstances which interfere significantly with the comfort health or welfare of persons or with the full use of enjoyment of property.
A typical legal definition of air pollution is the presence in the outdoor atmosphere of substances or contaminants put there by man, in quantities or concentrations and of a duration as to cause any; discomfort to a substantial number of inhabitants of a district of which are injurious to public health or to human, plant or animal life or property or which interfere with the reasonable comfortable enjoyment of life and property throughout the state or throughout such territories or areas of the state as shall be affected thereby (WHO).
From the above definitions, air pollution definition may be simplified as the presence of pollutants in air in sufficient quantity and duration which adversely affect the health and enjoyment of property of human beings, animals and plants.
Essay # 2. Classification of Air Pollutants:
ADVERTISEMENTS:
Air pollutants may be broadly classified which are as follows:
A. Based on the Origin of Pollutants:
(i) Primary Pollutants:
Primary pollutants are the pollutants which are emitted directly into the atmosphere from the sources, e.g. release of sulphur dioxide by burning of coal.
(ii) Secondary Pollutants:
Secondary pollutants are the pollutants which are formed due to the interaction of two or more primary pollutants or a result of some reaction with normal atmospheric constituents with or without photo activation, e.g. ozone (nitrogenoxide + O2 → O3).
B. Based on the Chemical Composition:
(i) Organic pollutants, e.g., Hydrocarbons
(ii) Inorganic pollutants, e.g., CO, SO2, SO3, H2S
C. Based on the State of Matter:
(I) Particulate Pollutants:
Particulate pollutants are finely divided solid or liquid particles. Particulates can be composed of inert or extremely reactive materials of size from 0.0002 (small molecule) to 500 microns, (1 micron = 10‑6m, e.g., dust, smoke, fumes etc.).
(II) Gaseous Pollutants:
Gaseous pollutants are the pollutants which are present in the form of gas e.g., SO2, H2S
Based on the size, the particulate matter may be classified which are as follows:
(i) Aerosols:
Aerosols are nothing but air suspensions. The particles suspended may be dust, smoke, mist, and fumes. This is a most general term applied to any tiny particles, either liquid or solids, dispersed in the atmosphere.
(ii) Dust:
It is an air suspension of irregular shaped mineral or other particles of size 1 to 200 microns. They settle under the influence of gravity. It is generated by crushing, chipping, grinding and by natural disintegration of rock and soil. Particles of this size range will be in the suspended state from, a few seconds to several months. If the size is less than 100 microns, it is called as fine dust and greater than 100 microns called as coarse dust. Particles of size greater than 50 microns can be seen with unaided eye. The typical sizes of dusts are given below.
(iii) Smoke:
It is an aerosol of very fine carbon particles of size range from 0.5 to 1.0 micron which are produced by incomplete combustion of organic particles such as coal, wood etc.
(iv) Soot:
Soot is agglomeration of carbon particles of size 1 to 10 microns impregnated with tar, formed due to incomplete combustion of carbonaceous materials.
(v) Fumes:
Fumes are the fine solid particles formed by condensation of gaseous state after volatilisation. The size range of particles is 0.03 to 1 micron.
(vi) Mist:
Mist is an aerosol of liquid droplets formed by the condensation of vapour. The size range of natural water vapour mists are from 40 to 500 microns. Usually the size is less than 10 µ.
(vii) Fog:
Water mist is called fog. It is the mist in which the liquid is water (sufficiently dense to obscure visibility). It is a visible aerosol.
(viii) Smog:
Smoke plus fog is expressed as smog.
(ix) Spray:
It consists of liquid droplets formed by the atomization of parent liquids (pesticides). The size of particles range from 10 to 1000 microns.
(x) Haze:
Haze is an air pollution condition (decrease in the visibility in atmosphere) formed due to presence of very fine dust, mist etc. in the atmosphere. Haze is expressed as Coefficient of Haze (COH) which is defined as –
(xi) Gas:
Gas is a matter which is having neither independent shape nor volume and tending to expand indefinitely.
(xii) Vapour:
Gaseous phase of matter which normally exist in a liquid or solid state.
Based on the chemical composition inorganic gaseous pollutants are classified as follows:
(i) Sulphur Compounds:
Coal, oil contains sulphur as impurity. Sulphur content of coal varies 1 to 5%. When fuel is burned, the sulphur also burns producing sulphur dioxide gas and also sulphur trioxide gas. They are the dominant oxides of sulphur present in the atmosphere.
SO2:
The most important oxide emitted by pollution sources is sulphur dioxide. It is a colourless, nonflammable, nonexplosive gas. It cause a taste sensation at concentrations from 0.3 to 1.0 ppm in air. At concentrations greater than 3 ppm, the SO2 gas has a pungent irritating odour.
It is partly converted to sulphur trioxide or sulphuric acid and its salts by photochemical or catalytic processes in the atmosphere. In a polluted atmosphere, SO2 reacts photochemically or catalytically with other pollutants. Sulphurdioxide gas alone can irritate the upper respiratory tract and it can be carried deep into lungs.
Sulphur Trioxide:
It is generally emitted along with sulphur dioxide (1 to 5 % concentration). SO3 rapidly combines with moisture in the atmosphere and form sulphuric acid. Both SO2 and SO3 are washed from the atmosphere during raining.
H2S (Hydrogen Sulphide):
Hydrogen sulphide is a foul smelling gas produced during anaerobic biological decomposition and also from kraft pulp plants, etc.
(ii) Nitrogen Compounds:
The oxides of nitrogen (NO, NO2) are the primary pollutants released by petroleum operations industrial and automobile combustion. There are seven oxides of nitrogen (N2O, NO, NO2, NO3, N2O3, N2O4, and N2O5) in which three oxides namely nitrous oxide (N2O), nitric oxide (NO) and nitrogen dioxide (NO2) are formed in the atmosphere. Generally NO and NO2 are analysed together and referred as NOx.
Nitrous Oxide (N2O):
Nitrous oxide is a colourless, odourless gas commonly present in the atmosphere (0.25 ppm in concentration), which is released due to biological activity of the soil. It is also called as laughing gas and anesthetic.
Nitric Oxide (NO):
It is also a colourless, odourless gas which forms under high temperature combustion processes. It is not a pollutant and inert at normal atmospheric condition. It is oxidised to nitrogen dioxide (NO2) in a polluted atmosphere through photochemical reaction. NO is emitted into the large quantities by automobiles than NO2. It is formed in combustion process with high temperature.
NO in atmosphere readily oxidised to NO2.
Nitrogen Dioxide (NO2):
It is a brown pungent gas with an irritating odour. It is corrosive to materials and toxic to man. It absorbs sunlight energy (ultra violet range) and initiates photochemical reactions that produce smog. It is emitted by fuel combustion and nitric acid plants. It is heavier than air and it is readily soluble in water which forms nitrous acid and nitric oxide.
Both combine with ammonia in the atmosphere and form ammonium nitrate and fall with rain.
(iii) Carbon Compounds:
Carbon monoxide is a primary pollutant which is colourless, odourless and tasteless gas. It is mainly originated from incomplete combustion of fuels (carbonaceous materials), especially due to automobile exhausts. It is highly poisonous gas and is generally considered as an asphyxiant. Carbon monoxide is present in small concentration in the atmosphere with a life time of 2 to 4 months. The toxicity of carbon monoxide is due to its affinity for haemoglobin which is the oxygen carrier of the blood. It is non-toxic to insects and other forms of life which have no red blood cells. CO has no detrimental effects on material surfaces and vegetation.
CO2:
Carbon dioxide is also produced along with carbon monoxide, but it is not considered as pollutant. Burning of fossil fuels such as coal, oil, and natural gas releases carbon dioxide, which is accumulating over the earth from the past 500 million years. These huge man-made emissions have been entering the atmosphere faster than the natural cycle (carbon dioxide → air and dissolved water → plants → animals carbon dioxide).
The temperature of the earth’s surface increases when the carbon dioxide in the atmosphere increases, because CO2 strongly absorbs long wave (Infrared) terrestrial radiation and continued CO2 build up, traps heat and prevents some of it to re-radiate back in to space.
In the natural cycle, carbon dioxide is uniformly distributed up to 75 km over the earth surface. About half of the extra carbon dioxide produced by combustion of hydrocarbons is retained in the atmosphere. As per the projections of the some investigators, by 2000 A.D. carbon dioxide increases 125 % of 1850 level, which is sufficient to produce significant changes in climate.
(iv) Hydrocarbons:
These are the primary pollutants released into the atmosphere in petroleum production and incomplete combustion of fuels like gasoline, diesel etc. The gaseous and volatile liquid hydrocarbons are the major air pollutants in the atmosphere. Their presence in air alone are not harmful. They undergo chemical reactions in the presence of sunlight and nitrogen oxides forming photochemical oxidants.
Derived hydrocarbons such as aldehydes, carbon tetrachloride etc. may be released as primary pollutants and also secondary pollutants in the certain conditions.
(v) Fluoride Compounds:
Fluorine is very toxic and corrosive substance when it is present in elemental state. Generally it is not present in atmosphere as air pollutant. But fluorides may be found in the atmosphere as gaseous or as a particulate matter.
All fluoride containing compounds are likely to be chemically reactive and in unpolluted atmosphere, the concentration of fluorides is very low. But, fluoride-emitting industries substantially contribute fluorides to the surrounding area. Industries such as fertilizer, aluminium, steel, etc. utilise fluoride-containing compounds and release as fluorides along with other waste gases in to the atmosphere.
Silicon tetrafluoride (SiF4) and gaseous hydrogen fluoride are the primary pollutants which are emitted mainly from fertilizer industry. Phosphate rocks which may contain 2-4% fluorides while processing with strong sulphuric acid, volatile hydrogen fluoride and silicon tetra fluoride gases are significantly emitted into the atmosphere. Calcium or sodium fluorides are used in steel industry, which are added to molten steel to release gaseous products. Fluorides will come out as gaseous effluents along with other exhausts.
Essay # 3. Causes of Air Pollution:
The smoke released in large quantities from vehicles and factories causes air pollution. Factories also give out poisonous chemicals, which mix with air. Burning of fuels at home and forest fire also contributes of air pollution.
For example, wind also causes air pollution by adding dust, bacteria and other particles into the air. We burn fuel at home for cooking. We burn coal in factories. Petrol or diesel is burnt as a result of combustion in vehicles. As a result of all these combustion carbon dioxide, carbon and carbon monoxide gases enter the atmosphere. Thus air gets polluted.
As a result of industrial revolution, numbers of industries have come up. The smoke that comes out of the chimney of these factories contains a large quantity of carbon monoxide, oxides of sulphur and nitrogen, etc. These are poisonous. When these gases enter the atmosphere, the air gets polluted. People living in industrial areas suffer from lung disease like tuberculosis, asthma, ear and eye disease also.
In rural parts wood, cow-dung, leaves, barks of trees is used as fuel. There is no proper ventilation in houses. The poisonous gases produced while burning cannot go out easily. So the ladies and children at home breathe this air. They become victims of a number of lung disease.
When we think of air pollution, we get the mental picture of factories with taller chimneys thick smoke that comes out of it. Equally dangerous are the crores of people who smoke cigarette, beedies, etc. you need not smoke. To be near them is enough.
The smoke enters the body; we get a number of lung diseases, the most dangerous lung cancer. Peepul trees are very common in villages. Scientists have investigated the advantages of Peepul trees. Peepul trees absorb a large quantity of carbon dioxide and gives out equal amount of oxygen. So we have to grow more Peepuls trees and take care to protect them.
Some children are fond of cutting down branches of trees and disfigure them. We must show interest in growing plants but not cutting down trees. It is true that the space available in cities is very less.
We can think of growing plants that occupy less space. We can grow some twiners or runners that spreads on our roofs. We can also grow plants in empty tins, pots and bottles. Horticultural departments will supply saplings tree. By economical use and reuse we can control air pollution.
Essay # 4. Forms of Air Pollution:
i. Indoor and Outdoor Air Pollution:
Indoor air pollution can be particularly hazardous to health as it is released in close proximity to people. It is stated that a pollutant released indoors is many times more likely to reach the lung than that released outdoors. In the developing countries a fairly large portion of the population is dependent on biomass such as wood, charcoal, agricultural residue, and animal waste for their energy requirements.
Open fires used for cooking and heating are commonly found in the household both in the rural and the urban areas. In addition, they are often not fitted with a chimney to remove the pollutants. In such households the children, women and animals, pets in particular, are most likely to be affected. The main pollutant in this environment is the SPM.
In fact, mortality due to indoor air pollution, mainly particulate matters, in the rural areas of India is one of the highest in the world. Many of the deaths are due to acute respiratory infections in children; others are due to cardiovascular diseases, lung cancer, and chronic respiratory diseases in adults.
If emissions are high and ventilation is poor, household use of coal and biomass can severely affect the indoor air quality. Household use of fossil fuel is also fairly common in the developing countries, particularly coal both bituminous and lignite.
These are particularly damaging as they burn inefficiently and emit considerable quantities of air pollutants. If emissions are high and ventilation poor, then the exposure levels to the gases emitted are far higher.
The most harmful of the gases and agents that are emitted are particulate matter, carbon dioxide, polycyclic organic matter, and formaldehyde. The indoor concentrations of these pollutants are far higher than the acceptable levels and are major concern in rural areas.
ii. Fly Ash:
Thermal power generation through coal combustion, amounting nearly 73% of India’s total installed power generation capacity, produces minute particles of ash called fly ash which cause serious environmental and health problems.
These ash particles consist of silica, alumina, oxides of iron, calcium, and magnesium and toxic heavy metals like lead, arsenic, cobalt, and copper. Most of the thermal power stations, cement and steel plants and railways in India use bituminous coal and produce up to 150 million tonnes fly ash annually.
This poses problems in the form of land use, health hazards, and environmental dangers to survival of man and animals. The prevalent practice is to dump fly ash on wastelands, and this has lain to waste thousands of hectares all over the country.
Fly ash interferes with the process of photosynthesis of aquatic plants and thus disturbs the food chain. Besides, fly ash corrodes exposed metallic structures in its vicinity. Being very minute, fly ash tends to remain airborne for a very long period causing serious health problems like irritation to eyes, skin, and nose, throat, and respiratory tract. Repeated inhalation of fly ash dust containing crystalline silica can cause bronchitis and lung cancer.
iii. Smog:
The word smog has been coined from a combination of the words fog and smoke and refers to hazy air that causes difficult breathing conditions. The term smog describes the conditions of fog mixed with smoke in it. Smog is a combination of various gases with water vapour and dust. Smog is produced when fuels are burnt and sunlight reacts with these gases and fine smoke particles in the air. Smog can affect outlying suburbs and rural areas as well as big cities.
Its occurrences are often linked to heavy traffic, high temperatures, and calm winds. During the winter, wind speeds are low and cause the smoke and fog to stagnate; hence pollution levels can increase near ground level. This keeps the pollution close to the breathing area near the ground. Heavy smog greatly decreases ultraviolet radiation.
During the early part of the 20th century, heavy smog in some parts of Europe resulted in a decrease in the production of natural vitamin D leading to a rise in the cases of rickets. The most harmful components of smog are ground-level ozone and fine airborne particles. Ground-level ozone forms when pollutants released from gasoline and diesel-powered vehicles and oil-based solvents react with heat and sunlight. It is harmful to humans, animals, and plants.
During the winter months, emissions of smoke and sulphur dioxide are much greater due to the burning of coal for heat in urban areas than they are during the summer months. Smoke particles trapped in the fog give it a yellow/black colour and this smog often settle over cities for many days.
The effects of smog on human health are evident, particularly when smog persist for several days. Many people suffer respiratory problems and increased deaths are recorded, notably those relating to bronchial causes. Relatively little is done to control any type of pollution or to promote environmental protection. Today, smoke and sulphur dioxide pollution in cities is much lower than in the past, as a result of legislation to control pollution emissions and cleaner emission technology.
iv. Carbon Pollution:
Carbon pollution is the release of tiny particles into the air from burning fuel for energy. Air pollution caused by such particulates has been a major problem since the beginning of the industrial revolution and the development of the internal combustion engine.
Mankind has become so dependent on the burning of fossil fuels (petroleum products, coal, and natural gas) that the sum total of all combustion-related emissions now constitutes a serious and widespread problem, not only to human health, but also to the entire global environment.
v. Acid Rain:
Another effect of air pollution is acid rain. The phenomenon occurs when sulphur dioxide and nitrogen oxides from the burning of fossil fuels such as, petrol, diesel, and coal combine with water vapour to form sulphuric and nitric acid in the atmosphere and fall as rain, snow or fog.
These gases can also be emitted from natural sources like volcanoes. Acid rain causes extensive damage to water, forest, soil resources and even animal and human health. It can corrode buildings and be hazardous to human health.
Because the contaminants are carried long distances, the sources of acid rain are difficult to pinpoint and hence difficult to control. For example, the acid rain that may have damaged some forest in Canada could have originated in the industrial areas of USA.
In fact, this has created disagreements between Canada and the United States and among European countries over the causes of and solutions to the problem of acid rain. The international scope of the problem has led to the signing of international agreements on the limitation of sulphur and nitrogen oxide emissions.
vi. Ozone Depletion:
There has been a slow and steady decline of about 4 percent per decade in the total amount of ozone in Earth’s stratosphere particularly a much larger, but seasonal, decrease in stratospheric ozone over Earth’s polar regions since around 1980. The latter phenomenon is commonly referred to as the ozone hole.
The most important process for this is catalytic destruction of ozone by atomic chlorine and bromine. The main source of these halogen atoms in the stratosphere is photo dissociation of chlorofluorocarbon (CFC) compounds, commonly called freons, and of bro-mofluoro-carbon compounds known as halons.
These compounds are transported into the stratosphere after being emitted at the surface. Ozone depletion mechanisms is strengthened due to enhanced emissions of CFCs and halons. CFCs, halons and other contributory substances are commonly referred to as ozone- depleting substances (ODS).
Since the ozone layer prevents most harmful UV wavelengths (270-315 nm) of ultraviolet light (UV light) from passing through the Earth’s atmosphere, projected decreases in ozone have generated worldwide concern leading to adoption of the Montreal Protocol banning the production of CFCs and halons as well as related ozone depleting chemicals such as carbon tetrachloride and trichloroethane (also known as methyl chloroform).
It is suspected that a variety of biological consequences such as increases in skin cancer, damage to plants, and reduction of plankton populations in the ocean’s photic zone may result as a consequence of increased UV exposure due to ozone depletion.
vii. The Greenhouse Effect:
The greenhouse effect was discovered by Joseph Fourier in 1824 and first investigated quantitatively by Svante Arrhenius in 1896. This is the process in which the emission of infrared radiation by an atmosphere warms a planet’s surface.
The Earth’s average surface temperature is about 30°C warmer than it would be without the greenhouse effect. In addition to the Earth, Mars and especially Venus have greenhouse effects. The Earth receives energy from the sun in the form of radiation. The Earth reflects about 30% of the incident solar flux; the remaining 70% is absorbed, warming the land, atmosphere and oceans.
To the extent that the Earth is in a steady state, the energy stored in the atmosphere and ocean does not change in time, so energy equal to the absorbed solar radiation must be radiated back to space. Earth radiates energy into space as black-body radiation, which maintains a thermal equilibrium. This thermal, infrared radiation increases with increasing temperature.
Thus earth’s temperature as being determined by the infrared flux needed to balance the absorbed solar flux. The visible solar radiation heats the surface, not the atmosphere, whereas most of the infrared radiation escaping to space is emitted from the upper atmosphere, not the surface. The infrared photons emitted by the surface are mostly absorbed by the atmosphere and do not escape directly to space.
In the earth’s atmosphere/the dominant infrared absorbing gases are water vapor, carbon dioxide, and ozone (O3). Carbon dioxide is a linear molecule, but it has an important vibrational mode in which the molecule bends with the carbon in the middle moving one way and the oxygen on the ends moving the other way, creating some charge separation by a dipole moment.
A substantial part of the greenhouse effect occurs due to carbon dioxide because this vibration is easily excited by infrared radiation. An increase in the concentration of carbon dioxide will provide more heat absorption and subsequently rise in the atmospheric temperature.
Global warming thus may have its implications as alteration in the climate and other factors which ultimately may result in ecological imbalance. Deforestation has aggravated the situation by reducing the conversion of CO2 and thus raising its atmospheric level. Other absorbers of significance include methane, nitrous oxide and the chlorofluorocarbons which are also increased in the atmosphere due to anthropogenic indiscriminate exploitation of natural resources.
Essay # 5. Effect on Vision Resulting in the Reduction of Visibility:
American Meteorology Society (Boston), defined the visibility as the greatest distance in a given direction at which it is just possible to see and identify with the unaided eye, (a) in a day time, a prominent dark object against the sky at the horizon and (b) at night, a known preferably unfocussed moderately intense light source.
Visibility is the maximum distance possible to see and identify with an unaided eye in a given direction. The visibility is reduced by scatter of sun light by particulates and is more when relative humidity is less than 70%.
Presence of submicron particles in the atmosphere with concentration of 150 micrograms of cubic meter can reduce visibility of 3.5 km. Hygroscopic particulates (particles that collect water from the atmosphere and form, a mist), carbon, tar, metal particles in the atmosphere also reduce visibility. Crystalline compounds like iron, aluminium, silicon, and calcium associate with sulphates, nitrates, chlorides, and fluorides also reduce visibility.
Particulate concentrations of 100,000/cm3 can reduce visibility to 1.6 km. NO2 at 0.25 ppm will cause reduction of visibility and at 8 to 10 ppm, it reduces visibility up to 1.6 km. NO2 absorbs light and causes the sky to appear brownish in colour. Visibility is greatly effected by concentrations of Sulphur dioxide more than 100 micrograms/m3 and particles in the size range of 0.1 to 1.0 microns particulates and SO2 of 150 micrograms/m3 with relative humidity of less than 70% can reduce visibility up to 8 km.
Concentration of 0.1 ppm of SO2 with a significant concentration of particulate matter and relative humidity of 50% can also reduce visibility to about 8 km. Solid and liquid particles in the submicron range from 0.1 to 1.0 micron are responsible for the decrease in visibility.
Particulate concentration of 750 µg/m3 accompanied by 715 µg/m3 of sulphur dioxide of 24-h average can increases the illness. Children likely to experience increased incidence of respiratory diseases for concentration of 120 µg/m3 of particulate matter and sulphur dioxide with annual mean concentration.
To determine the visibility the following formula may be adopted.
V = 5.2 T r / (K · C)
Where,
V = visibility, km
T = density of particles, kg/m3
r = radius of particles, micrometers
K = scattering area ratio, dimensionless
C = concentration of particles, micrograms per m3
This is not valid when the humidity is greater than 70%.
Essay # 6. Sources of Air Pollution:
Air pollution is largely confined to the troposphere or lower atmosphere, with some penetration into the stratosphere. Air is never found absolutely clean in nature. Air pollution started from the very moment when the primitive man knew to make fire. Air pollutants are substances causing damage to target or receptor. The target may be man, animal, plant, building or material which may be adversely affected by pollutants.
Major sources of air pollution may be grouped into two:
1. Natural Sources such as Volcanic eruptions releasing poisonous gases like SO2, H2S, CO etc., forest fires, natural organic and inorganic decays, marsh gases, depletion of sand and dust, cosmic dust, aero-allergens or pollen grains of flowers, soil debris, and fungal spores etc. All these produced are injurious to health. Green plants through evaporation release huge amount of CO2. The effect of CO2 in increasing the earth’s temperature is commonly referred to as “Greenhouse Effect”.
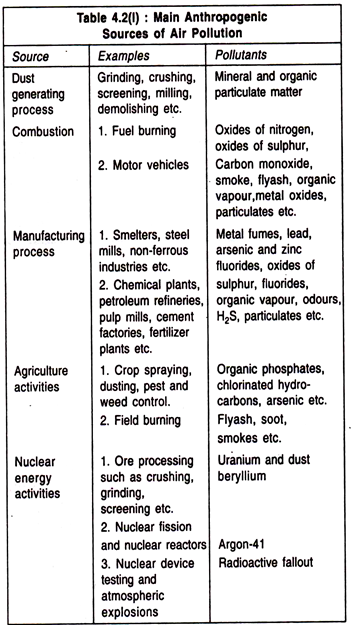
2. Anthropogenic Sources such as increase in population, deforestation, burning of fossil fuels and fires, emissions from vehicles, rapid industrialization, agricultural activities and wars are the major causes of air pollution. Principal anthropogenic sources of air pollution are represented in Table 4.2(I).
Essay # 7. Air Pollution in India:
The World Health Organization estimates that about two million people die prematurely every year as a result of air pollution, while many more suffer from breathing ailments, heart disease, lung infections and even cancer. Fine particles or microscopic dust from coal or wood fires and unfiltered diesel engines are rated as one of the most lethal forms or air pollution caused by industry, transport, household heating, cooking and ageing coal or oil-fired power stations.
There are four reasons of air pollution – emissions from vehicles, thermal power plants, industries and refineries. The problem of indoor air pollution in rural areas and urban slums has increased. India’s environmental problems are exacerbated by its heavy reliance on coal for power generation. Coal supplies more than half of the country’s energy needs and is used for nearly three-quarters of electricity generation. While India is fortunate to have abundant reserves of coal to power economic development, the burning of this resource, especially given the high ash content of India’s coal, has come at a cost in terms of heightened public risk and environmental degradation.
Reliance on coal as the major energy source has led to a nine-fold jump in carbon emissions over the past forty years. The government estimates the cost of environmental degradation has been running at 4.5% of GDP in recent years. The low energy efficiency of power plants that burn coal is a contributing factor. India’s coal plants are old and are not outfitted with the most modern pollution controls.
With pollution level rising across the country, India on Wednesday, the 18 November 2009 revised the national ambient air quality standards after 15 years. “We have notified the ambient air quality standards in India which is equivalent to the European level and exceeds the standard prevalent in the US,” Minister of state for environment and forests Jairam Ramesh said.
The revised ambient air quality standards provide a legal framework for the control of air pollution and the protection of public health and any citizen can approach the court demanding better air quality.
Vehicle emissions are responsible for 70% of the country’s air pollution. The major problem with government efforts to safeguard the environment has been enforcement at the local level, not with a lack of laws. Ai0072 pollution from vehicle exhaust and industry is a worsening problem for India. Exhaust from vehicles has increased eight-fold over levels of twenty years ago; industrial pollution has risen four times over the same period. The economy has grown two and a half times over the past two decades but pollution control and civil services have not kept pace. Air quality is worst in the big cities like Kolkata, Delhi, Mumbai, Chennai, etc.
Bangalore holds the title of being the asthma capital of the country. Studies estimate that 10 per cent of Bangalore’s 60 lakh population and over 50 per cent of its children below 18 years suffer from air pollution- related ailments.
Chennai:
Exhaust from vehicles, dust from construction debris, industrial waste, burning of municipal and garden waste are all on the rise in the city. So are respiratory diseases, including asthma. At least six of the 10 top causes of death are related to respiratory disease, says Dr. D. Ranganathan, director (in-charge), Institute of Thoracic Medicine.
Mumbai:
Not only are levels of Suspended Particulate Matter above permissible limits in Mumbai, but the worst pollutant after vehicular emissions has grown at an alarming rate. The levels of Respirable Suspended Particulate Matter (RSPM), or dust, in Mumbai’s air have continued to increase over the past three years.
The air pollution in Mumbai is so high that Mumbai authorities have purchased 42,000 litres of perfume to spray on the city’s enormous waste dumps at Deonar and Mulund landfill sites after people living near the landfill sites complained of the stench. The Deonar landfill site, one of India’s largest, was first used by the British in 1927. Today, the festering pile covers more than 120 hectares and is eight story’s high.
These cities are on the World Health Organization’s list of top most polluted cities. Vehicle exhaust, untreated smoke, and untreated water all contribute to the problem. Continued economic growth, urbanisation, and an increase in the number of vehicles, together with lax enforcement of environmental laws, will result in further increases in pollution levels. Concern with New Delhi’s air quality got so bad that the Supreme Court recently stepped in and placed a limit on the number of new car registrations in the capital.
The effects of air pollution are obvious – rice crop yields in southern India are falling as brown clouds block out more and more sunlight. And the brilliant white of the famous Taj Mahal is slowly fading to a sickly yellow. In the famous “Taj Mahal Case” a very strong step was taken by Supreme Court to save the Taj Mahal Case being polluted by fumes and more than 200 factories were closed down.
Wind patterns during the monsoon waft air pollutants from India high into the atmosphere to pollute the stratosphere, scientists have said in a fresh study on March 24, 2010 blaming Asia for distant air pollution. An international research team has combined satellite observations and computer models to suggest that monsoon circulation serves as an effective pathway to carry pollution from India and South Asia into the stratosphere.
Essay # 8. Prevention and Control of Air Pollution
:
Actually, prevention of air pollution is not so simple. It is impracticable to provide at reasonable cost. All the growing needs and amenities of modern life are causing some air pollution. In other words, air pollution has become a necessary evil of development. For example, it is difficult to drive a car or scooter without causing some air pollution. Similarly, it is difficult to run an industry without causing some air pollution. It is not possible to run a thermal plant without fouling the atmosphere.
It is, however, possible to prevent air pollution without undue cost by:
(i) Careful planning and setting of industries.
(ii) Better design equipment, and
(iii) Better operation of the equipment.
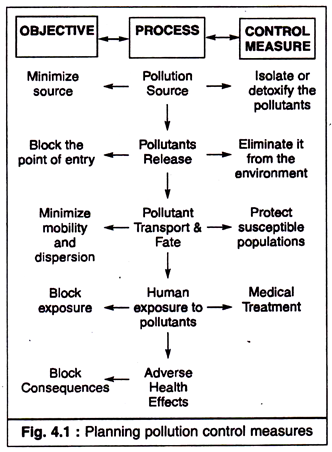
The objective of air pollution control should be to reduce the harm of a potential air pollutant. This is a multi-step process (Fig. 4.1), which indicates an effective air pollution control programme.
If a pollutant is spotted to be potentially hazardous based on the results of toxicological assessments, its control should genuinely be attempted. It is not uncommon, at least with highly carcinogenic environmental agents, that a modification of the human desire (or wants) initiates an effective pollution control programme. If man is not stringent enough to continue the use of a particular chemical despite its threatening qualities against him and his environment, pollution control is simple — ‘Find an alternative’.
Technological advancement has always been able to come up with almost everything that man has desired. But if there is no alternative to a chemical in use then efforts need be undertaken to control its entry into the environment.
The air pollutant can either be isolated at the point of release or its production and converted for another purpose — thus keeping it within restricted areas; if that chemical (example — a by-product or a final product) is highly toxic and not useful, then its release into the environment has to be blocked and the chemical be destroyed by specific processes in restricted areas. A systematic pollution control would begin with a search for an alternative for a process or a substance in use, if adequate control measures cannot be taken to prevent the entry of the substance into the environment.
General Methods of Air Pollution Control:
Following are the general methods of controlling air pollution:
1. Zoning.
2. Air pollution control at source.
3. Installation of controlling devices and equipment.
4. Air pollution control by high stacks or chimneys to discharge the pollutants at higher altitudes.
5. By planting trees and growing vegetation.
The zoning of the industries is done based upon the type of industries, their function etc. If zoning is done properly, it results in considerable improvement of health of the community as a whole. It prevents the invasion of undesirable industries in and around residential areas and so toxic, hazardous, and harmful gases and odours are prevented from entering or attacking the humans living in residential areas.
The industries running on electric power and causing no such nuisance may be allowed to be set close to the residential zone. The industries causing nuisance and producing undesirable gases and odours and other deleterious by-products may be located far away from the city in spacious lands.
2. Air Pollution Control at Source:
The air pollution problem can be minimized at the source by making use of the following measures:
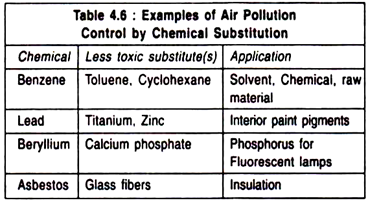
(i) Raw Material Substitution:
In order to reduce air pollution, it is desirable to substitute the raw material (chemical) if it results in pollution by another one, which is less polluting (Table 4.6):
(ii) Redesigning Equipments or Changing the Operational Products:
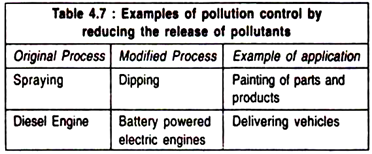
Release of toxic substances into the environment from a manufacturing unit or due to usage patterns can also be controlled.
Toxic emissions can be reduced by redesigning equipments or changing the operational procedure (Table 4.7):
In some cases, equipment alterations such as the use of floating roof tanks rather than vented tanks can cut down the evaporation losses. New type of equipment, for example the basic O2 furnaces which are replacing the open hearth furnaces in steel industry, pose much less air pollution problems.
3. Installation of Controlling Devices and Equipment:
Because of large number of industries, different types of gases are released into the atmosphere along with particulates and become major source of air pollution. In order to prevent these pollutants into the environment, control devices have been used depending on the collection property and capacity of the device and nature of the process used by the particular industry.
Based on the method of removal three important types of dust collectors have been used:
(i) Internal separators
(ii) Wet collection devices
(iii) Electrostatic precipitators
(i) Internal Separators:
These separate the dust particles present in the gases. These are manufactured in various sizes and shapes.
The commonly used internal separators are:
(a) Gravity settling chamber
(b) Cyclones
(c) Fabric filters.
(a) Gravity Settling Chambers:
These comprise of large chambers in which dust is separated from the gas by reducing the velocity of the gas. As a result, dust particles settle down in the chamber (Fig. 4.2). Coarse particles are thus removed. Gravity settling chambers are capable of removing only the large sized particles in the range of 25-30 μm diameter. The settling time required for smaller particles is so long that the use of this type of equipment is impractical in such cases.
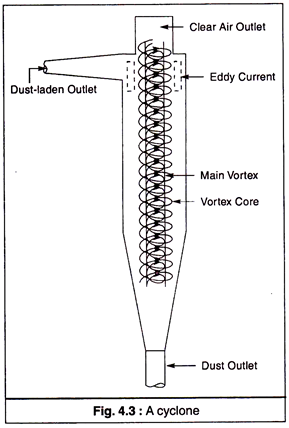
(b) Cyclones:
The principle of cyclone collector (Fig. 4.3) is that when a gas travels in a double vortex, the particles contained in it are separated.
A cyclone collector consists of a tight circular spiral that produces centrifugal force, as a result of which the suspended particles in the flowing gas are forced to move outward to a wall where they are collected. This process removes all small particles (diameter 5-30 µm) efficiently.
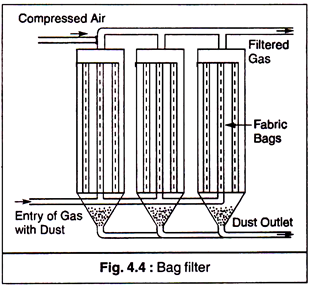
(c) Fabric Filters:
In fabric filters the gas with the dust is allowed to pass through a fabric to which the dust gets attached. If the gas is flowing at low velocity and contains considerable amount of large particulates, these settle down as a result of sedimentation. Due to electrostatic charges, fine particles are also attached to the fabric.
Bag filter (Fig. 4.4) is a device of removing the dust particles from the waste gases of the industrial processes. It is in the form of a tubular medium made of woven or felted fabric. These bags are fitted with hopper at bottom. The gas enters from the bottom through the hopper. The diameter of the bag is 1 m and height is about 7 to 10 m. Its collection efficiency is about 99%.
The heavy particles are settled down in the hopper due to gravity. Finer particles are deposited on the fabric of the bag. The bags are cleaned by blowing compressed air in the reverse direction. It is necessary to have low gas velocities of the order of 1-3 meter per minute.
(ii) Wet Collection Devices:
In such devices, a liquid is utilized to assist in the removal of solid, liquid or gaseous contaminants. The effectiveness of these devices depends primarily upon the frequency of contact and the degree of interaction between the liquid phase and the contaminants to be removed.
In precise form, devices using mixed phases of gases and liquid are known as wet washer and scrubbers. These are the collection devices in which the particles are washed out of the gas flow by a water spray. The aim is to transfer suspended particulate matters in the gas to the scrubbing liquid which can be readily removed by the gas cleaning device. This leaves the gas clean to pass onwards to the process for which it is being used, or alternatively to be discharged to the atmosphere.
Extreme care should be taken to see that the collected waste water does not become a source of water pollution. It may need special settling tanks, chemical flocculators or filtration units.
The most common wet collectors in use are:
(a) Cyclonic Scrubbers which have an efficiency of 90% and can remove dust particles of 5.0 µm size. It is capable of cleaning about 2,000 liters of gas per minute.
(b) Venturi Scrubbers which have efficiency of about 99% and can remove very fine particles. It can clean about 4,000 liters of gas per minute.
(iii) Electrostatic Precipitators:
Particles having diameters as small as 0.0001 cm can be removed using electrostatic precipitators. The principle involved in it is that when the particulates move through a region of high electric potential, they become charged and then they are attached to an oppositely charged area where they are collected and removed.
The electrostatic precipitators consist of a series of plates which are charged to high voltages, alternatively + and –. Particles approaching given plate tend to acquire its charge. They are then attracted to the surface of the next plate, from which they fall into the hopper below. Thus particles pickup charge as they pass between plates and are precipitated on plates of opposite charge.
The potential across the plates is around 50,000 volt. The high voltage in the wires produces billions of electrons and bombards the gas molecules, which become -vely charged. The +ve ions return to the -ve end electrode and gain electrons while the -ve ions combine with the dust particles and make them -vely charged. The negatively charged dust particles collect at the positively charged plates.
The efficiency of electrostatic precipitators is 99.9% and it is capable of cleaning 150,000 liters of gas per minute at a temperature of 600°C.
4. Air Pollution Control by Stacks:
It is a well-known fact that when the quantity of pollutants released is much and the air possesses a limited capacity of absorption, then the pollution becomes serious. If the pollutants are carried away to long distances or taken to high altitudes, they get reduced in concentration by diffusion or/and dilution. Diffusion of a pollutant in air actually depends mainly upon atmospheric temperature, speed and direction of wind.
Keeping the above factors in view, the pollutants are taken to high altitudes by means of stacks. The height of chimney stack should be raised to such a height that when the residual particulates are dispersed, they get spread evenly over a wide area. So the height as well as the diameter of the stack required is designed to keep the ground level concentration within permissible limits.
5. Air Pollution Control by Planting Trees and Growing Vegetation:
In order to reduce the spreading of air pollutants coming out from industrial sources, planting of pollution-resistant tress and growing vegetation around the industry has been recommended.
In addition to above methods, the odours of gases can be absorbed by passing them through beds of activated charcoal or sand or soil. The odours can also be controlled by the oxidation of the odorous compounds using Cl2, O3 and H2O2 as oxidizing agents.
The smoke envelops the indoor environment and several steps such as cleaner fuels, good quality stoves and better ventilation may be taken to reduce indoor or domestic air pollution.
Indigenous Air Pollution Control Equipments:
Pollution control is now a big business in India. Indian companies are now manufacturing a wide variety of air pollution control equipments including mechanical clones, dust collector/cyclones, electrostatic precipitators, fabric filters, wet collectors and venturi scrubbers.
Environmental Scientists at the NIST (National Institute of Standards and Testing) in Maryland (USA) have developed an instrument that can detect gases present in minute amounts. This instrument uses microwaves — similar to those used in microwave cookers — to measure as little as 10 ppb of any volatile organic or inorganic matter.
The sample gas is pulsed through a nozzle into a detection cavity where it is cooled down to -272°C. At this temperature, molecules can be distinguished from one another through their light absorption patterns. The device can be used to monitor automobile emissions as well as indoor air quality.


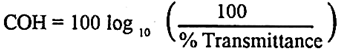
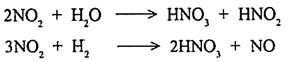
![]](https://www.shareyouressays.com/wp-content/uploads/2018/04/clip_image00214_thumb2_thumb.png)